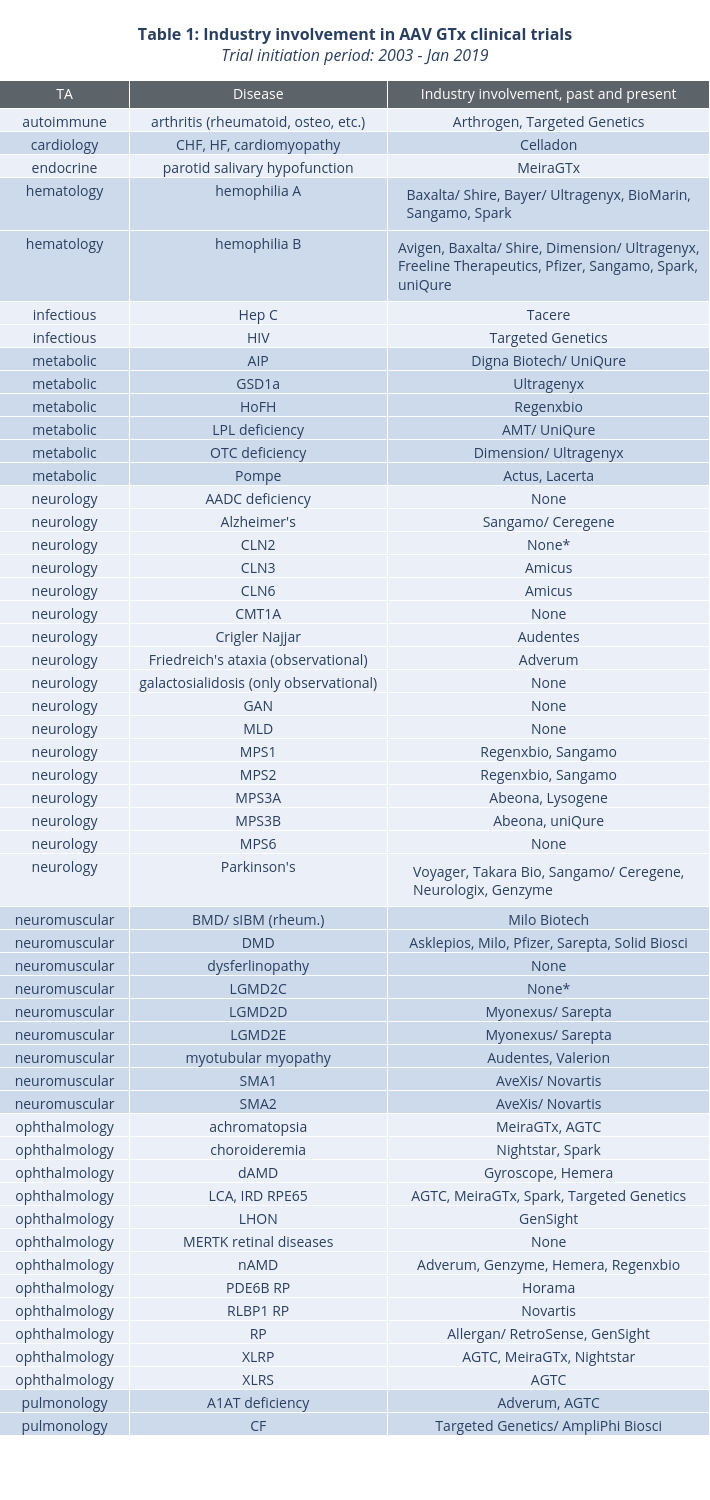
 Industry sponsors in AAV gene therapy clinical trials
Industry sponsors in AAV gene therapy clinical trials
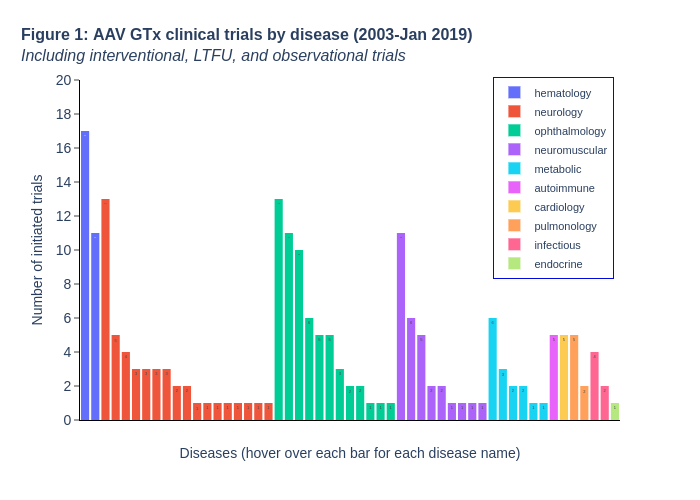
 AAV gene therapy clinical development progress
AAV gene therapy clinical development progress
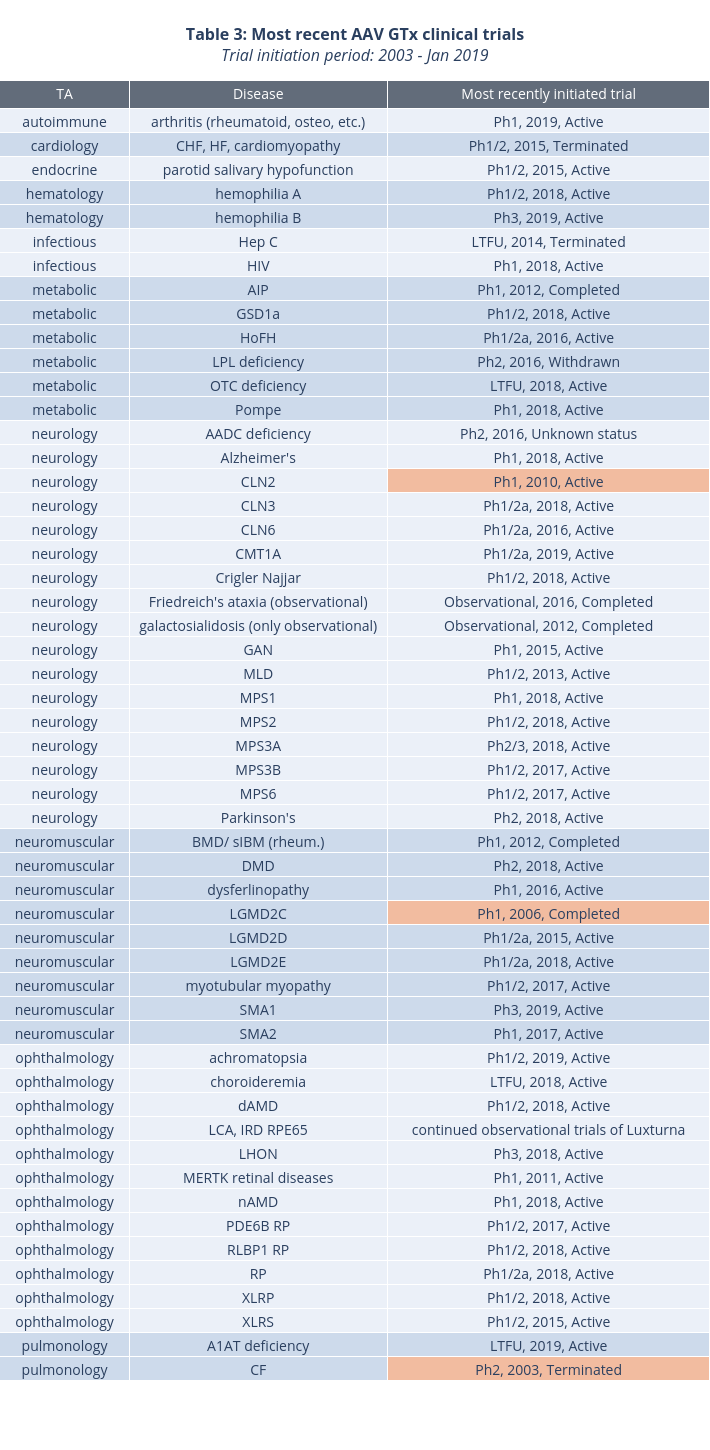
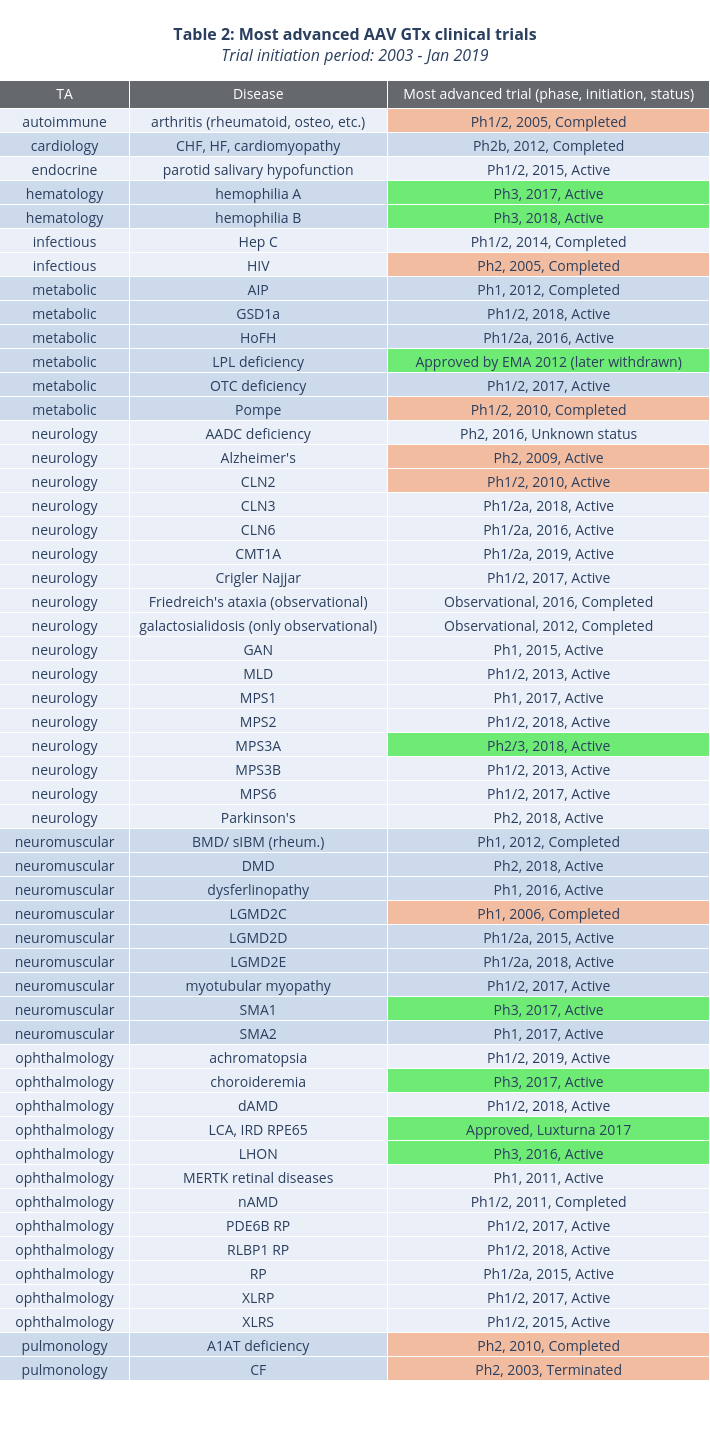 Finally, we looked at the most recently initiated trials for each disease, through January 2019, to gauge the level of interest in pursuing specific indications. Table 3 summarizes the results. In red are trials that have been initiated over eight years ago: CLN2, LGMD2C, and cystic fibrosis (CF). However, preclinical progress in these conditions reported to date (ASGCT 2019 and various press releases) indicates that clinical development will likely continue in the near future. Of these three conditions, cystic fibrosis has already been targeted by variety of non-AAV gene therapy approaches, including mRNA (NCT03375047) and antisense oligonucleotides (NCT02564354, NCT02532764, NCT03647228).
Finally, we looked at the most recently initiated trials for each disease, through January 2019, to gauge the level of interest in pursuing specific indications. Table 3 summarizes the results. In red are trials that have been initiated over eight years ago: CLN2, LGMD2C, and cystic fibrosis (CF). However, preclinical progress in these conditions reported to date (ASGCT 2019 and various press releases) indicates that clinical development will likely continue in the near future. Of these three conditions, cystic fibrosis has already been targeted by variety of non-AAV gene therapy approaches, including mRNA (NCT03375047) and antisense oligonucleotides (NCT02564354, NCT02532764, NCT03647228).
 Thank you for reading! We will be updating this analysis annually or biannually. Please subscribe to make sure you do not miss the next update.
Thank you for reading! We will be updating this analysis annually or biannually. Please subscribe to make sure you do not miss the next update.
