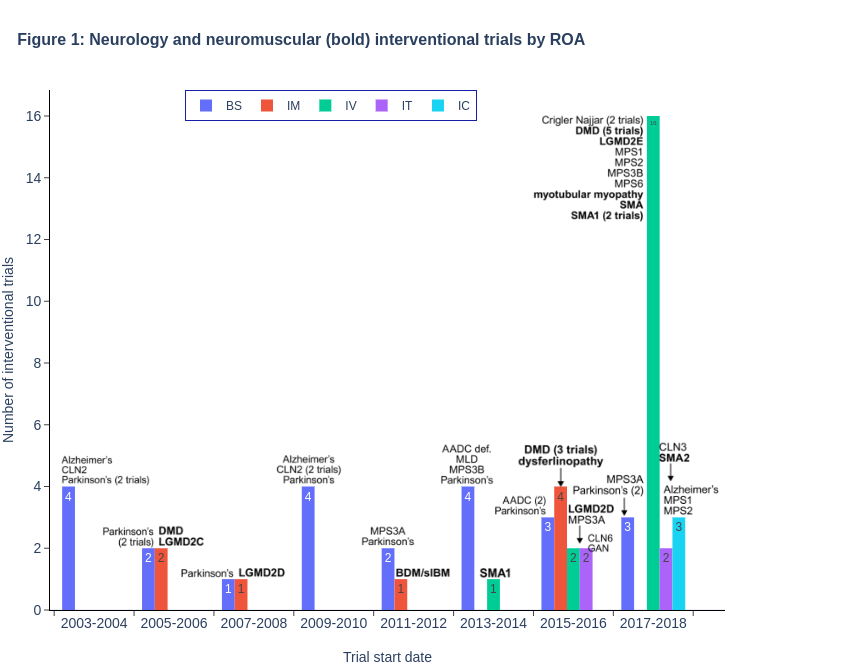
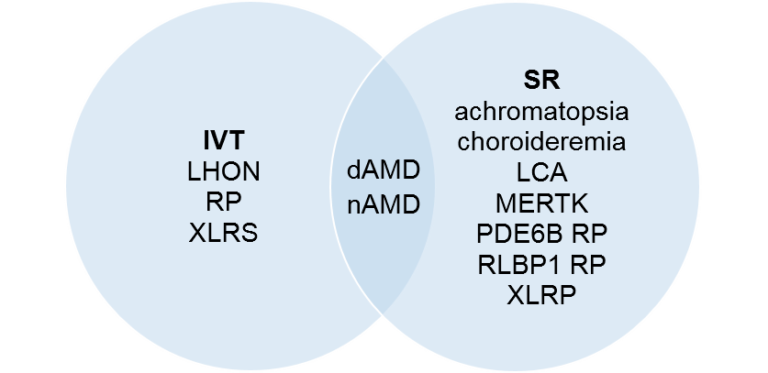
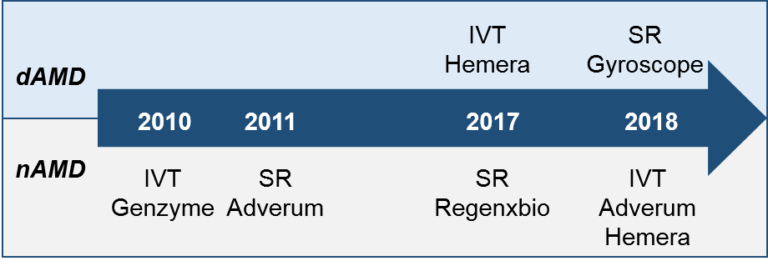
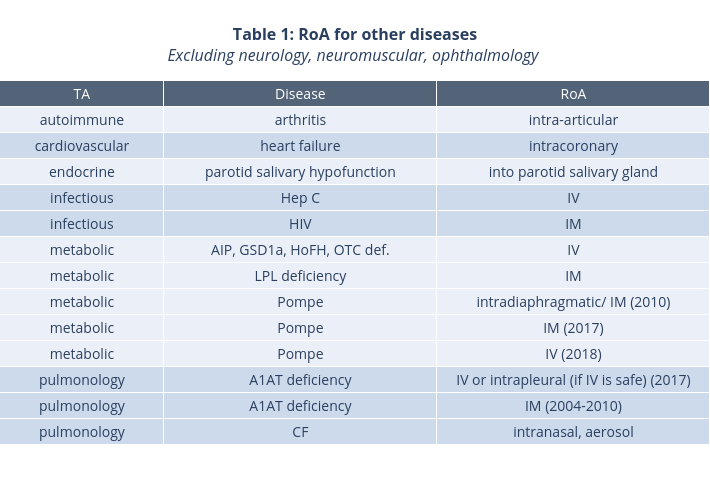
 If you are interested in the entire dataset of clinical trials and corresponding RoAs, please check out our downloadable file in Store. Also, we provide significant discount for current and former BioHeights clients, as well as non-profit and academic organizations.
If you are interested in the entire dataset of clinical trials and corresponding RoAs, please check out our downloadable file in Store. Also, we provide significant discount for current and former BioHeights clients, as well as non-profit and academic organizations.
